SUMMARY
A two-step chemical process using a special reagent to swap a ketone's oxygen atom with a sulfur atom creating new compounds, particularly useful for making improved drugs
The Unmet Need: Efficient strategies for achieving precise functional group modifications of bioactive compounds for novel drug development
- In the realm of synthetic chemistry, particularly for drug discovery and lead optimization, there is a continuous need for versatile methods to modify existing chemical structures. Incorporating different atoms, such as sulfur or nitrogen, into bioactive molecules can significantly enhance their pharmacological properties, including potency, selectivity, and metabolic stability. This ability to precisely alter molecular architecture is crucial for exploring new chemical space and developing more effective therapeutic agents.
-
Current synthetic methodologies face significant challenges in achieving such precise atom swaps, especially for replacing carbonyl groups with other heteroatoms. Existing approaches often necessitate laborious and inefficient de novo syntheses, requiring the molecule to be built from scratch rather than directly modified. Furthermore, some transformations rely on the use of highly toxic and environmentally hazardous stoichiometric mercury reagents, which are impractical and undesirable for industrial applications. These limitations mean that many desired structural modifications are either difficult, costly, or virtually impossible to achieve through conventional means.
The Proposed Solution: Novel reagents for directly replacing the carbonyl group in ketones with other atoms with high yield and selectivity
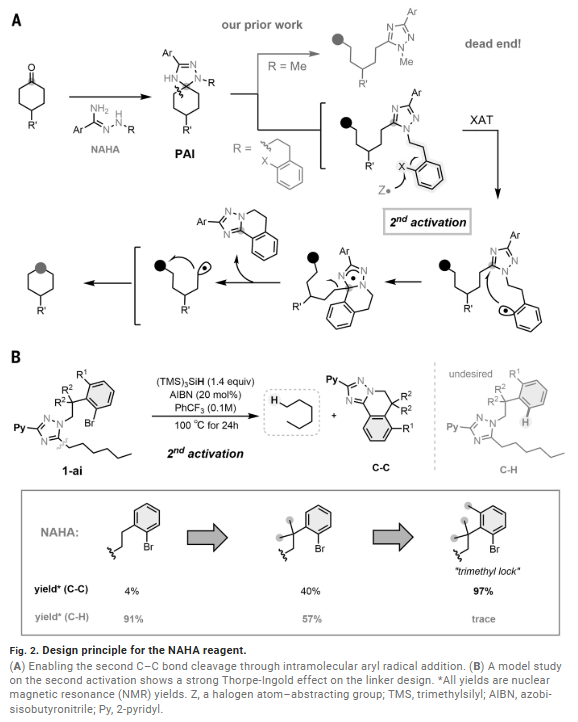
- The faculty inventor enabled the atom swapping of carbonyl groups in ketones, specifically replacing a ketone carbonyl with a sulfur atom. This two-step, redox-neutral method utilizes a rationally designed bifunctional N'-alkyl-hydrazonamide (NAHA) reagent containing an aryl iodide moiety. The process involves ketone condensation with NAHA, forming a pre-aromatic intermediate. This intermediate undergoes a first carbon-carbon bond cleavage, with the resulting radical trapped by a sulfur atom transfer reagent. The subsequent thiolate undergoes intramolecular cyclization, driven by a silyl radical-mediated halogen-atom transfer, initiating a second carbon-carbon bond cleavage and intramolecular sulfur trapping to form a cyclic thioether.
-
The platform is highly differentiated, representing a novel transformation that addresses significant limitations in synthetic chemistry. Unlike conventional methods requiring inefficient de novo syntheses or toxic reagents, this approach provides direct access to novel heteroatom-containing analogs, particularly sulfur derivatives, previously difficult or impossible to synthesize. Its core innovation lies in the rationally designed NAHA reagent system, enabling sequential double carbon-carbon bond activation through a unique halogen-atom transfer mechanism. This streamlined, redox-neutral route offers significant advantages for drug discovery and lead optimization, as sulfur incorporation can dramatically enhance biological activity.
FIGURE
ADVANTAGES
ADVANTAGES
-
Enables novel atom swapping of ketone carbonyls (e.g., to sulfur, nitrogen), providing access to previously inaccessible analogs
-
Offers a streamlined, redox-neutral, and efficient synthetic route, overcoming limitations of traditional, often hazardous, methods
-
Demonstrates broad substrate scope and excellent chemoselectivity, compatible with diverse functional groups.
-
Significantly enhances drug discovery and lead optimization by creating analogs with potentially improved biological activity.
APPLICATIONS
- Drug discovery
- Lead optimization
- Research tools
- Bioactive molecule modification
PUBLICATIONS
July 10, 2025
Proof of concept
Patent Pending
Licensing,Co-development
Guangbin Dong