SUMMARY
-
Sepsis is a systemic response to infection and remains a leading cause of death in intensive care units worldwide while our understanding of the pathogenesis of sepsis across most tissues and organs of the body remains rudimentary. The disease is a global health issue in need of targeted therapies addressing the short- and long-term effects on the host.
-
A myriad of cells and molecules has been linked to sepsis. Numerous studies have established immune and endothelial cells together with cytokines and the complement and coagulation systems as key cellular and molecular factors in the pathogenesis of sepsis. The uncontrolled, systemic activity of cytokines contributes to tissue injury and organ failure.
-
Moreover, recent studies have shown the release of a secreted phospholipase from the gut, PLA2G5, leads to hemolysis and failure of multiple organs introducing another mediator of sepsis. PLA2G5 release into the systemic circulation during sepsis leads to a concurrent increase in hemolytic activity in the blood, hemophagocytosis in the spleen, and heme levels in the blood, the latter being linked to multi-tissue injury.
-
The faculty inventor developed an antibody blockade method targeting PLA2G5 to decrease intravascular hemolysis. In vivo studies demonstrate that PLA2G5 blockade rescues mice from lethal sepsis.
FIGURE
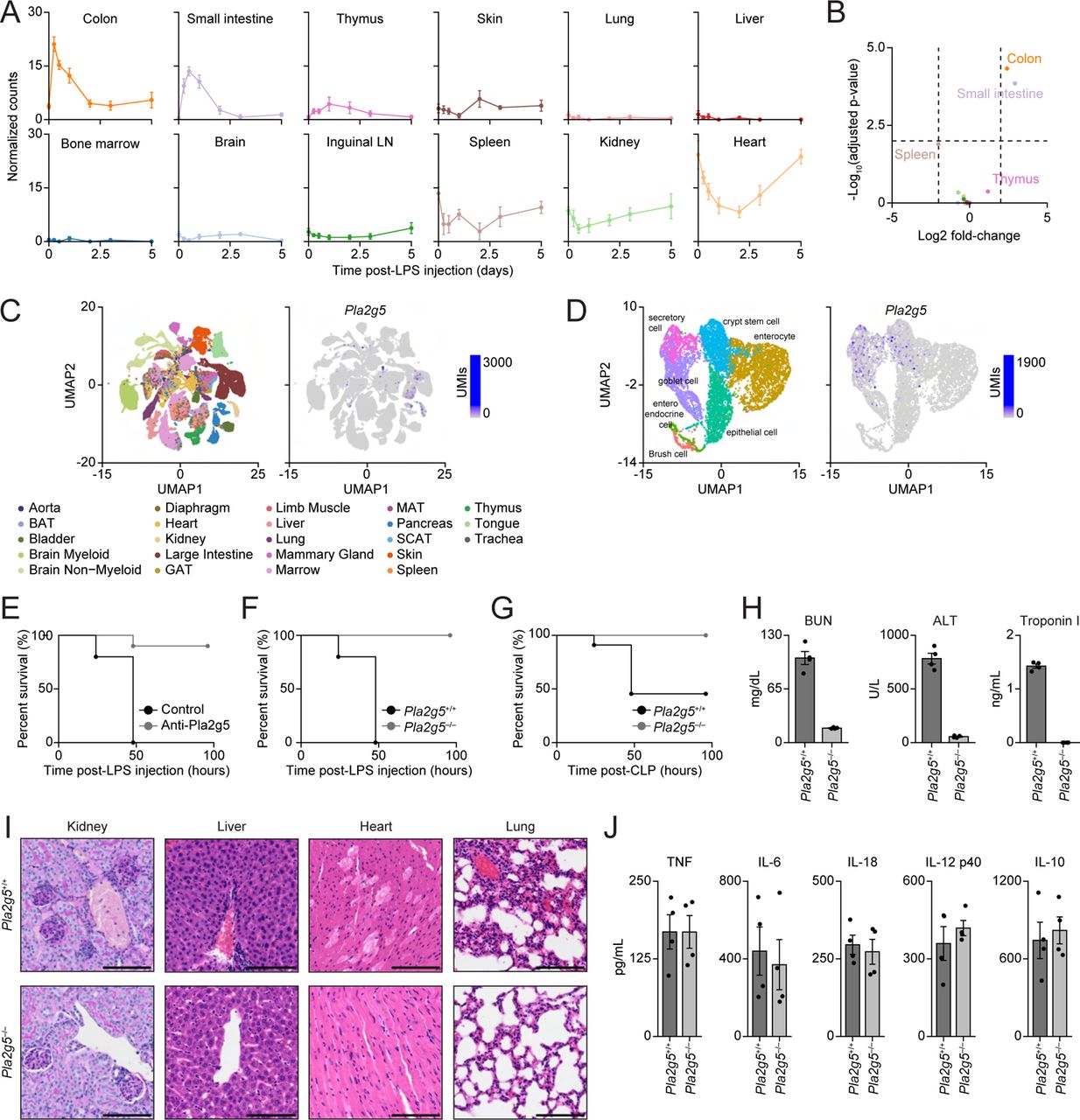
Functional Perturbations of PLA2G5 Promotes Survival to Sepsis
(A) Normalized counts for Pla2g5 from indicated organs. LN, lymph node; Error bars, SEM (n = 3-4). (B) Volcano plot for Pla2g5 expression at 6 hours post-LPS injection across all 12 organs from A. (C-D) Single-cell expression of Pla2g5 mRNAs across mouse tissues. Shown are uniform manifold approximation and projection (UMAP) plots of Pla2g5 mRNA counts (left panels; C-D) across indicated mouse organs (right panel; C) or large intestine cell types (right panel; D). BAT, brown adipose tissue; GAT, gonadal adipose tissue; MAT, mesenteric adipose tissue; SCAT, subcutaneous adipose tissue. (E-G) Survival curves of mice upon LPS (E-F) or CLP (G) sepsis using ©2G5 neutralizing antibodies (E) or indicated mouse genotypes (F-G) (n = 10-11). (H) Serum levels of indicated organ injury markers at 24 hours post-injection of a sublethal dose of LPS in indicated mouse genotypes. Error bars, SEM (n = 4). (I) Histological analysis of kidney (PAS staining) and liver, heart, and lungs (H&E staining) from Pla2g5+/+ (top) or Pla2g5-/- (bottom) mice at 24 hours post-sublethal LPS injection. (J) Serum levels of indicated cytokines (top) in Pla2g5+/+ or Pla2g5-/- mice at 24 hours after LPS injection. Error bar, SEM (n = 4). See also Figure S6 and Table S5.
ADVANTAGES
ADVANTAGES
- Reduces multi-organ damage from inflammation
APPLICATIONS
- Therapeutic intervention for sepsis
- Diagnostic to monitor sepsis
- Research tool
PUBLICATIONS
-
Organism-Wide Analysis of Sepsis Reveals Mechanisms of Systemic Inflammation. Michihiro Takahama, Ashwini Patil, Katherine Johnson, Denis Cipurko, Yoshimi Miki, Yoshitaka Taketomi, Peter Carbonetto, Madison Plaster, Gabriella Richey, Surya Pandey, Katerina Cheronis, Tatsuki Ueda, Adam Gruenbaum, Steven M. Dudek, Matthew Stephens, Makoto Murakami, Nicolas ChevrierbioRxiv 2023.01.30.526342; doi: https://doi.org/10.1101/2023.01.30.526342
June 3, 2024
Proof of concept
Patent Pending
Licensing,Co-development
Nicolas Chevrier