SUMMARY
A novel drug delivery system using exosomes derived from hippocampus neuron cells engineered to express the Fe65 protein targeting amyloid-β precursor protein to reduce amyloid plaques Alzheimer's disease. This system crosses the blood-brain barrier, enhances uptake by neuronal cells, and induces autophagy, improving cognition and reduce disease pathogenesis.
The Unmet Need: Effective therapeutics and delivery systems to treat Alzheimer's Disease pathogenesis and progression
- Alzheimer's disease (AD) research focuses on understanding and developing treatments for this progressive neurodegenerative disorder, which primarily affects memory and cognitive function. AD is characterized by the formation of amyloid plaques, neurofibrillary tangles, and the overexpression of the amyloid-β precursor protein (APP) in the brain. Current therapeutic approaches, such as acetylcholinesterase inhibitors and NMDA receptor antagonists, only provide symptomatic relief or slow disease progression.
-
Traditional medicine has also been explored for its potential in cognitive enhancement. However, the need for more effective treatments that can directly target the pathological mechanisms of AD remains unmet. One promising area of research involves using exosomes as drug delivery vehicles due to their ability to cross the blood-brain barrier and deliver therapeutic agents to specific brain regions affected by AD.
-
Current approaches to treating AD face several challenges. Despite the potential of exosomes as drug delivery vehicles, conventional methods often result in poor brain permeability and limited bioavailability of therapeutic compounds. For instance, compounds like Corynoxine-B (Cory-B), which are known to induce autophagy and reduce amyloid-β levels, face difficulties in crossing the blood-brain barrier when administered alone.
-
Additionally, while exosomes can be engineered to target specific proteins like APP, the natural interaction between APP and its binding partners, such as Fe65, complicates targeted delivery. Traditional exosome-based delivery systems may also accumulate in non-target organs like the liver and spleen, reducing the efficiency of brain targeting. These limitations highlight the need for innovative approaches to enhance the delivery and efficacy of therapeutic agents in AD treatment.
The proposed solution: A novel exosome-based targeted drug delivery system with engineered hippocampus neuron cell-derived exosomes for targeted delivery of an autophagy inducer to disrupt plaque formation
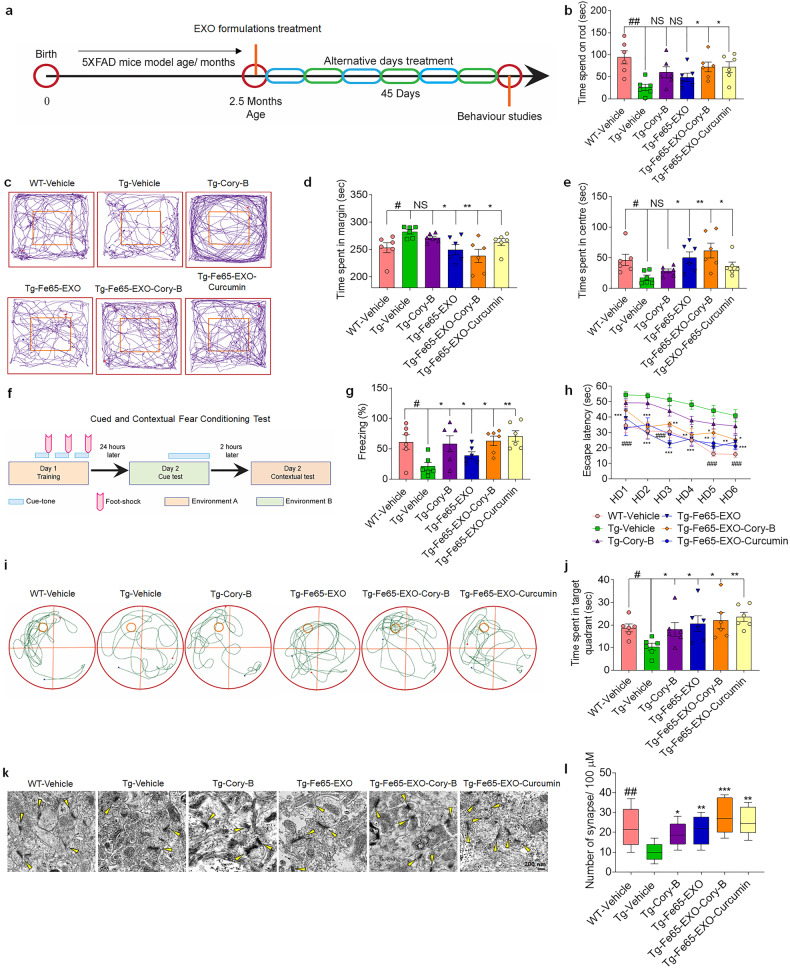
- The faculty inventor developed a sophisticated drug delivery system designed to target AD. This system utilizes exosomes derived from hippocampal neuron cells, which are engineered to express the Fe65 protein on their surface. These Fe65-engineered exosomes (Fe65-EXO) encapsulate Corynoxine-B (Cory-B), a compound known for inducing autophagy.
-
The primary function of this system is to target the amyloid-β precursor protein (APP) in the brains of AD patients, thereby disrupting the harmful interaction between APP and Fe65, which is crucial in the formation of amyloid plaques.
-
The Fe65-EXO facilitates the delivery of Cory-B across the blood-brain barrier, enhancing its uptake by APP-overexpressing neuronal cells and inducing Beclin-1 dependent autophagy. This approach has been demonstrated to improve cognitive function and reduce AD pathogenesis in mouse models, showing promise as a therapeutic intervention.
FIGURE
ADVANTAGES
ADVANTAGES
- Minimalized off-target effects
- Penetrance of blood brain barrier for treatment of AD and neurological conditions
- Reduces AD pathogenesis
- Validated amelioration of cognitive decline in murine models
APPLICATIONS
PUBLICATIONS
-
Iyaswamy A, Thakur A, Guan XJ, Krishnamoorthi S, Fung TY, Lu K, Gaurav I, Yang Z, Su CF, Lau KF, Zhang K, Ng RC, Lian Q, Cheung KH, Ye K, Chen HJ, Li M. Fe65-engineered neuronal exosomes encapsulating corynoxine-B ameliorate cognition and pathology of Alzheimer's disease. Signal Transduct Target Ther. 2023 Oct 23;8(1):404. doi: 10.1038/s41392-023-01657-4. PMID: 37867176; PMCID: PMC10590775.
July 17, 2024
Proof of concept
Patent Pending
Licensing,Co-development
Joyce Chen