SUMMARY
The technology enhances cytokine signaling and immune function, optimizing the antiviral and anti-cancer efficacy of Type III IFNs by adjusting cytokine-receptor interaction affinity and relative JAK geometry, providing an effective treatment for cancer and viral infections with reduced side effects.
The unmet need: Due to limited efficacy and toxicity concerns, the use of therapeutic cytokines has been superseded or relegated to second-line therapy by advanced immunotherapeutics
- The field of cytokine signaling plays a crucial role in the regulation of immune responses, including antiviral and anti-cancer activities. Type I Interferons (IFNs), for instance, are widely used for treating a range of viral infections and cancers due to their potent immune-modulatory properties. However, their use is often limited by severe side effects, primarily due to the ubiquitous expression of their receptors.
-
Type III IFNs were developed as a less toxic alternative due to their tissue-specific receptor expression, particularly in the lungs and liver. Despite their lower toxicity, Type III IFNs are less effective in their immune responses, necessitating further advancements to enhance their efficacy while maintaining their reduced side effect profile.
-
Current approaches to improving cytokine signaling have encountered significant challenges. While some strategies have focused on altering the affinity between cytokine receptors and Janus kinases (JAKs), these methods have typically resulted in increased sensitivity (lower EC50 values) but have not substantially enhanced the overall efficacy or maximum response (Emax) of the signaling.
-
Additionally, the toxic effects of Type I IFNs remain a major concern. Efforts to optimize the geometry of intracellular JAK interactions have shown promise but are still in the early stages of development. Moreover, no existing engineered cytokines or molecules have successfully optimized both the affinity and geometry of these interactions, leading to suboptimal signaling and function. This underscores the need for innovative solutions that can simultaneously address these multifaceted challenges to improve therapeutic outcomes for viral infections and cancer.
The proposed solution: Optimized cytokine signaling to amplify antiviral and anticancer efficacy while reducing the toxicity observed in Type I interferons
-
The faculty inventor, Juan Mendoza, established a method to develop compounds for cytokine signaling modulation through two main mechanisms: altering the affinity of cytokine-receptor JAK interactions and adjusting the relative geometry of intracellular JAKs. By optimizing these factors, it significantly enhances the signaling and functional potency of cytokines, which has notable antiviral and anti-cancer effects.
-
Specifically, this technology shows that type III IFNs, which have limited efficacy under natural conditions, can be engineered to achieve a performance nearly equivalent to the highly potent but toxic type I IFNs. The tuning of both the extracellular receptor complex affinity and JAK-JAK geometry is key to achieving these improvements, laying the foundation for developing molecules that can modulate these interactions dynamically to either enhance or diminish the receptor-JAK interactions and their associated functions.
FIGURE
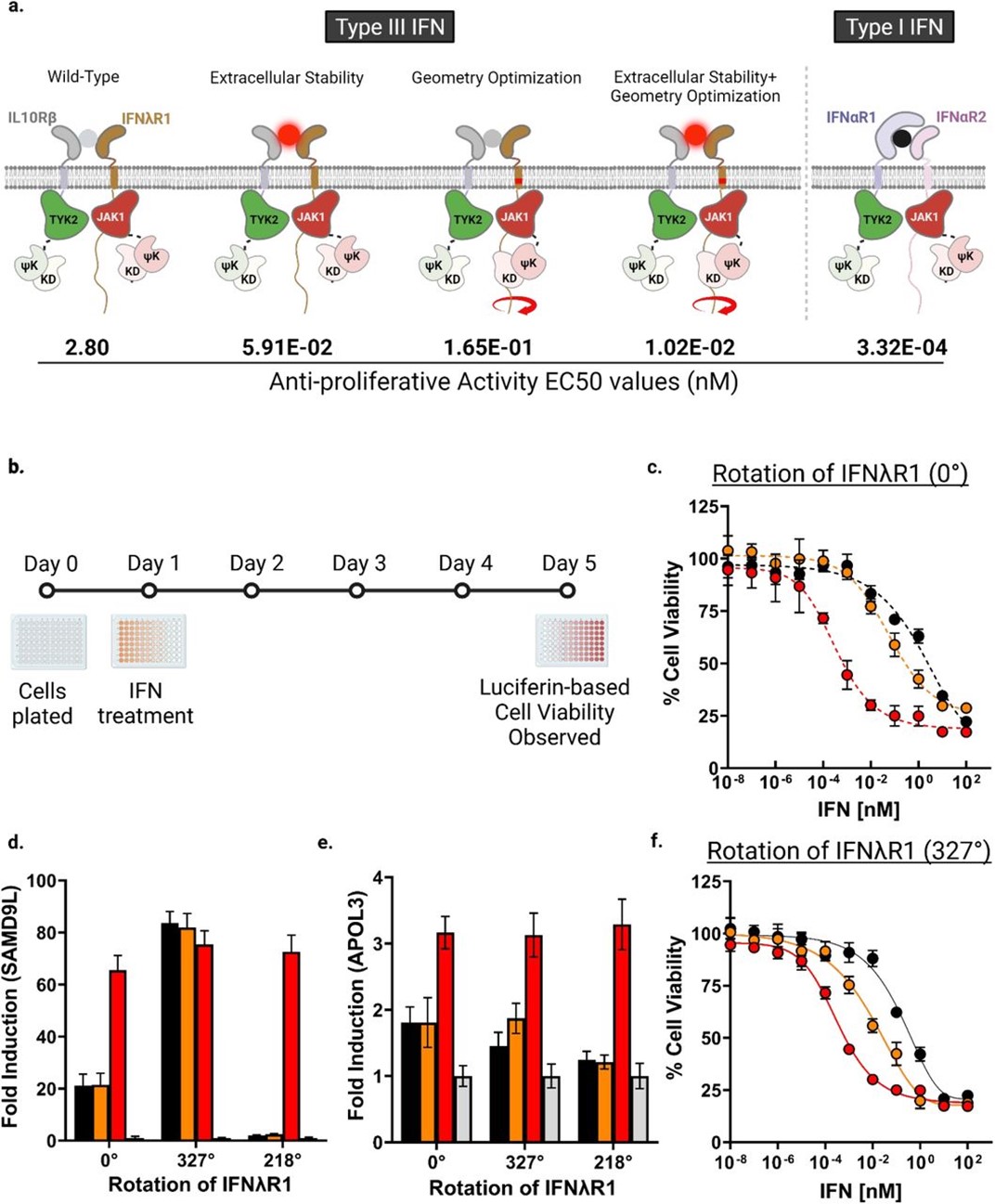
Optimization of IFNλR1 upregulates anti-proliferative activities of type III IFNs.
a, Schematic diagram summarizing the optimization strategies and their respectively associated EC50 values (nM) of the anti-proliferative assay and calculated fold-changes relative to IFNω treated wild-type IFNλR1 expressing cells (assigned value 1). b, schematic diagram showing the experimental set-up of the assay. c, Anti-proliferative activity of IFNs in cells expressing either the wild-type or f, optimized IFNλR1. Cells were incubated with serial dilutions of IFNλ3 (black), H11 (orange) or IFNω (red) for 4 days. Curves were fit to a first-order logistic model. Error bars represent ± SEM (n=3). d, PCR quantification of fold changes in induction of SAMD9L, e, APOL3 at 6h post treatment with 100nM each of IFNλ3 (black), H11 (orange), or IFNω (red) in wild-type or optimized cell lines. Mean changes ± SEM in gene expression were determined relative to untreated cells (grey, assigned value of 1) and normalized to 18S (n=4).
ADVANTAGES
ADVANTAGES
-
Enhances antiviral and anti-cancer properties of cytokines
-
Optimizes tissue-specific efficacy, reducing toxicity
-
Allows alteration of cytokine signaling potency
-
Modulates receptor-JAK interactions to improve or decrease signaling
APPLICATIONS
- Immunotherapy
- Oncology
- Antivirals
- Gene Therapy
PUBLICATIONS
- William S. Grubbe, Fabian Byléhn, Walter Alvarado, Juan J. de Pablo, Juan L. Mendoza,
Molecular analysis of the type III interferon complex and its applications in protein engineering,
Biophysical Journal, Volume 122, Issue 21, 2023, Pages 4254-4263, ISSN 0006-3495, https://doi.org/10.1016/j.bpj.2023.09.021.
-
Enhanced Complex Stability and Optimal JAK Geometry are Pivotal for a Potent Type III Interferon Response. Theint Aung, Curt M. Horvath, Juan L. Mendoza. bioRxiv 2022.09.27.508945; doi: https://doi.org/10.1101/2022.09.27.508945
- October 16, 2024
Proof of concept
Patent Pending
Licensing,Co-development
Juan Mendoza